The modern environment is still as hostile to humans as it was centuries ago. And such terrible diseases as tuberculosis, polio, rubella, whooping cough, tetanus, etc. are especially dangerous for people.
Vaccines help save you from them - antigenic material; use as part of a vaccination schedule allows you to prevent infection with certain diseases or mitigate its negative consequences.
Such prevention, carried out with the help of special biological serums, forms immunity in the human body against many dangerous diseases. Therefore, great importance is attached to vaccines: after all, even the highest quality drug quickly loses its potency if it is placed in inappropriate conditions.
Consequences of violating the required storage conditions
- All immunobiological serums are protein substances that are extremely sensitive to the destructive effects of high temperatures.
- If vaccines are allowed to overheat, then their main component - living cells of viruses or microbes - is damaged and even killed. And these vaccinations become not only useless, but even harmful to human health - especially when used to vaccinate a child.
- If the adsorbed whey was subjected to freezing/thawing during storage, then its reactogenicity increases significantly. And then the introduction of the vaccine is fraught with the appearance of allergic reactions, local edema and other side effects. And overheating or freezing of immunoglobulin leads to the fact that after its administration a person’s systolic and diastolic blood pressure decreases.
- Freezing solvents entails a violation of the integrity of the ampoules, which is visually imperceptible, but subsequently causes serious changes in the properties of the contents. Vaccination with low-quality MIBP can lead to increased body temperature, convulsions, neurological disorders, insomnia, deterioration of the gastrointestinal tract system, rashes, joint pain, and enlarged lymph nodes.
Gam-Covid-Vac (trademark "Sputnik V")
Registered in Russia and more than 30 countries around the world, an application for registration in the EU has been submitted, and an application for WHO approval has been submitted.
This is a genetically engineered vector based on two strains of live human adenoviruses.
It is administered twice with an interval of 3 weeks.
The effectiveness is 91.4%, against severe disease - 100%.
It is expected that immunity is formed for two years (for 9 months - has already been proven).
Vaccinated people may experience a flu-like syndrome - fever (sometimes up to 38-39 degrees), muscle and joint pain, weakness, headache. If necessary, it is recommended to take antipyretic drugs. Symptoms usually go away within 1-2 days. Less commonly observed are nausea, dyspepsia, loss of appetite, and sometimes enlarged regional lymph nodes.
Who is it recommended for, contraindications, features
Used in adults 18-60 years old, use is also allowed for people aged 60+.
Contraindicated in pregnant and lactating women; studies are planned on children under 18 years of age.
Do not use if you are hypersensitive to any component of the vaccine; history of severe allergic reactions; acute infectious diseases, exacerbation of chronic ailments. The vaccination can be done 2-4 weeks after recovery or remission. For mild ARVI - after normalization of temperature.
In case of severe complications after administration of the first dose (anaphylactic shock, convulsive syndrome, temperature above 40 C, etc.), administration of the second component is also prohibited.
Can be used with caution in chronic liver and kidney diseases, diabetes mellitus, severe diseases of the hematopoietic system, epilepsy, strokes and other diseases of the central nervous system, a history of myocardial infarction, cervical cancer, primary and secondary immunodeficiencies, autoimmune diseases, lung diseases, asthma and COPD.
Storage equipment
According to SP 3.3.2.1248-03, to maintain a high level of activity of vaccines, only appropriate equipment - medical refrigerators - can be used.
- Outpatient clinics and clinics (primary care institutions) use small counter freezers and conventional 2-chamber freezers.
- In district medical institutions, vaccines are stored in household 2-chamber refrigerators (freezers).
- Hospitals at the regional level use freezers for cold cells, rooms and large counter freezers - the doors are located at the top.
- In clinical institutions at the national level, it includes freezing rooms, large counters opening from above.
In order to protect vaccines during their transportation, a thermal container and its version - a vaccine bag - are used. For the transportation of large quantities of MIBP, if storage is necessary in extreme situations or during the next defrosting, thermal containers that maintain a temperature of 0°C to +8°C for a long time are excellent.
Bags serve the same role, but for small quantities.
The indicators are monitored using sensors and different types of indicators - an indicator on the vaccine container, a freezing indicator, and a control indicator card.
Main types of thermometers:
- mercury or alcohol, optimal for any criteria;
- dial or pointer, fixing min/max t° values in the refrigerator/freezer compartment;
- liquid crystal, very convenient for temperature control in thermal containers;
- thermographs that record temperature on a strip of paper; they are necessary for system control in a specific period of time.
The chambers provide an optimal temperature range from +2°C to +8°C, suitable for most types of vaccines. The specified temperature range must be scrupulously observed at all stages of storage and transportation of vaccine sera.
In every medical institution, it is strictly necessary to register the receipt and/or departure of immunobiological drugs, and the following information must be strictly recorded:
- a copy of the MIBP production certificate;
- number of vaccines;
- series numbers and copies of certificates of conformity;
- release date and expiration date of serums;
- FULL NAME. employee responsible for these activities.
The health worker appointed by the order is obliged to note the temperature indicators and storage temperature in a separate log 2 times a day; it must be placed in the middle of the middle shelf. All medical institutions must have a clear emergency action plan in force majeure situations.
Impeccable fulfillment of these requirements is ensured by the so-called. cold chain is a specially organized government structure, the key components are:
- A system of strict control over the delivery and application processes.
- Trained personnel provide medical services, transport and store serums, and maintain equipment.
- High-quality refrigeration equipment, which includes, in addition to medical freezers and thermal containers, indicators.
It should be especially noted that the presence of even the most technologically advanced equipment cannot guarantee a stable level of functioning of this chain. Its effectiveness depends on the professionalism of health workers, their sense of responsibility, knowledge and skills.
The effectiveness of immunoprophylaxis depends on many factors:
- is the selection of persons for vaccinations carried out correctly (rejection of vaccination at elevated temperatures, the presence of acute conditions, exacerbation of chronic diseases);
- Are the instructions for diluting MIBP, determining the dose, and methods of administration strictly followed;
- Are contraindications to vaccination taken into account, etc.
Where are vaccination compounds stored?
In manufacturing plants and other warehouses, drugs are stored in refrigerators and freezers or rooms. Pharmacies use refrigerated display cases, household refrigerators, and freezer counters.
Transportation of drugs is carried out only on special transport equipped with a system for maintaining the set temperature. For transportation and temporary storage, thermal containers and thermal bags with cold elements are used.
In the refrigerator, vaccines are arranged in the following order:
- lyophilized drugs are stored on a shelf next to the freezer;
- the middle shelves are used for adsorbed and inactivated killed vaccines;
- On the shelf furthest from the freezer there are solvents for lyophilized drugs, as well as live ones (these groups of immunological drugs are prohibited from freezing). An exception is oral polio drops;
- shelves on the door and the bottom of the refrigerator are prohibited areas for storing any immunobiological agents.
The refrigerator should be no closer than 10 cm to the wall, and a thermometer must be installed in it. When defrosting the refrigerator, immunobiological preparations are transferred to a container with a cold element, and it is important to prevent their contact in order to avoid freezing of the preparations.
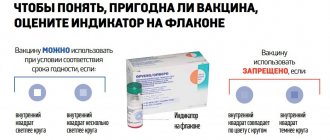
The ampoules in which immunobiological agents are produced are equipped with a thermal indicator to assess the temperature conditions in which the vaccine was on its way to users.
These may be indicators of freezing or rising temperatures. The symbols on the lid indicate the possibility of further use of the solution.
Correct connection of the refrigerator
When connecting, you need to take into account a number of nuances:
- compliance with the location where the equipment is located;
- distance to the walls of the room and nearby furnishings;
- state of the socket, the refrigeration unit will be connected;
- availability of thermometers.
When choosing a place for a refrigerator, you should be guided by the main goal - to strictly follow a number of rules. From this point of view, the most preferable is a separate room equipped with a modern ventilation system. It is also necessary that heating devices and any heat sources are located at a sufficient distance from these chambers.
The placement of the refrigerator is considered to comply with cold chain standards if it is placed on a level surface. Therefore, when installing it, use a building level. The unit is placed at a distance of 10 cm from the walls, which will ensure normal air circulation.
Before you start using the refrigerator, you need to make sure that the outlet is working properly and eliminate the possibility of accidentally disconnecting the unit from the power grid.
The readings make it possible to determine the boundaries of the permissible temperature range for the MIBP, so this device is the most important element of the control system. One thermometer should be installed in the refrigerator compartment, the second in the freezer.
Principles for storing MIBP in the refrigerator:
- according to SP 3.3.2.1120-02, boxes with vaccines sorted by type are placed in the main compartment on the middle and top shelves, and separately from each other (at a distance of 3-4 cm), a layer of cooled air is maintained between them;
- liquid vaccines such as Infanrix are stored exclusively on the bottom shelf; oral polio vaccine and lyophilized MIBP should be on top, but not very close to the evaporator;
- it is prohibited to place on door shelves, since the temperature there is not low enough;
- any others (Infanrix, DPT, Pentaxim, etc.) are recommended to be stored in the refrigerator for only three calendar months;
- expired MIBP should be disposed of rather than kept inside;
- if it is necessary to save them for observation, they are marked and moved to another refrigerator;
- to maintain a constant temperature, spare cold cells or plastic vessels with water are placed in the chamber;
- freezing of cold elements (or ice cubes) is carried out in the chamber itself;
- It is strictly forbidden to keep food and drinks together with the MIBP;
- You are allowed to open the door no more than 2-3 times a day, without breaking the rules.
Vaccines and the cold chain: maintaining the cold chain
The Infectious Disease Bulletin focuses on factors influencing the quality of the Dutch national vaccination program [ 1 ]. The conditions for transporting the vaccine from the manufacturer to the final consumer are very important. This path is usually called the “cold chain”. If the chain is too “weak,” it may break, rendering the vaccine ineffective. The World Health Organization's extensive Immunization Program has for many years focused on maintaining the cold chain, mainly in tropical and subtropical countries. Thus, in Nigeria, differences in seroconversion rates after measles vaccination at two vaccination centers in Nigeria were found to be largely due to vaccine handling [2]. In the 80s, this type of research began to be carried out in Europe. An inventory study was conducted in Holland in 1990 [3]. In the UK, a survey of family doctors found that in many cases the refrigerator used to store vaccines was not equipped with a thermometer and that multi-dose vials were often used for much longer than 24 hours, sometimes up to a month after opening [4]. Therefore, a limited study of vaccine use in 33 Dutch consultation offices was carried out. It turned out that most vaccinating authorities have problems with storing vaccines [5]. Based on the results of this study, “Recommendations for handling vaccines” were compiled; In the spring of 1993, a “Cold Chain Symposium” was held.
Why cold chain?
Vaccines are heat labile products and must be handled with care to maintain their properties. During the preparation of vaccines, requirements are strictly observed to ensure consistently high quality of the final product. These requirements are known as GMP (good manufacturing practice). One of these requirements relates to the storage conditions of vaccines at the manufacturer. Once the vaccines are handed over to the recipient, he becomes responsible for their proper storage and/or further transportation in the case of, for example, supplying a wholesaler or provincial vaccination organizations. Vaccines should be stored in their original packaging in a dark place at a temperature between 2 and 80°C. Only in this case is the preservation of their quality guaranteed until the expiration date indicated on the packaging. Each deviation from these requirements damages the quality of the vaccine and shortens the period of possible use.
Effects of too high temperatures When a vaccine is exposed to too high temperatures, the protein structure of the antigens can be disrupted, leaving less antigenic material in the vaccine. Live vaccines reduce the viability and infectivity of weakened pathogens, which are necessary for a good immune response. After using vaccines that were stored at too high temperatures, immunity will be insufficient or completely absent.
Consequences of too low temperatures
Lyophilized vaccines themselves can be preserved without damage at too low temperatures, but the diluent may become contaminated as microscopic cracks may appear in the wall of the bottle due to the expansion of the freezing liquid. The contents of these bottles cannot be used as a diluent for lyophilized vaccines. When storing liquid vaccines, the same problems arise as when storing solvents. In addition, in adsorbed vaccines such as DSP and DXP, freezing may destroy the bond with the adsorbent. The formation of flakes is typical; the vaccine cannot be shaken until homogeneous. Adsorption affects the effectiveness of the vaccine; after freezing, the immunogenicity of the vaccine is greatly reduced. Due to breaks in the cold chain, thousands of guilders worth of vaccines are lost every year. In these cases, it does not make sense to determine the effectiveness of the vaccines in all affected lots, since this would be significantly more expensive than replacing the vaccines. It is easy to assume that cases of breaking the “cold chain” are not always noticed. In such cases, vaccination is carried out, but immunity does not occur, and then an unfair conclusion may be made that the vaccine has failed. This alone makes good vaccine storage organization very important. This article examines the practical aspects of maintaining a “cold chain” and provides advice on organizing vaccine storage. Recommendations are given on how to handle vaccines if there is a break in the cold chain. Our advice can be used not only by those who carry out the state vaccination program, but also by vaccine sellers and everyone who vaccinates.
Vaccine stock: organization and responsibility
Each organization that owns vaccines should have a written protocol for storing vaccines and ensure that one person is responsible for storing vaccines. This person is responsible for enforcing storage regulations and can be called upon in the event of a disaster.
Fridge
A large stock of vaccines (for example, a bulk shipment) is usually stored in a large refrigerated facility, often custom-built and equipped with electronic monitoring and control systems. A smaller supply of vaccines (for example, available from a pharmacy or vaccination provider) can be stored in a regular home refrigerator. It is advisable that this refrigerator does not have a freezer compartment and an automatic defrosting system. If there is a freezer, there is a high probability that the vaccine may freeze in some areas. In addition, a refrigerator without a freezer can be defrosted and cleaned faster. An auto-defrost refrigerator regularly raises the temperature to allow frozen liquid to melt and drain. These frequent temperature fluctuations have a negative impact on the quality of stored vaccines. It is better to defrost the refrigerator yourself periodically. It is recommended to have two refrigerators for storing vaccines. One refrigerator should be in the reception area to store a day's supply. This refrigerator has to be opened regularly, which leads to unwanted temperature fluctuations, but this is not so significant since its contents are used up quite quickly. Another refrigerator is used to store supplies and it is advisable to open it no more than once a day. If you have several refrigerators, you must ensure that they are not connected to the same electrical group; If one fuse blows, the entire supply is lost. Do not store other items in the refrigerator that contains vaccines and medications. since in this case the refrigerator will open more often, which again will lead to unwanted temperature fluctuations. In addition, storing food in the refrigerator increases the likelihood of contamination and microbial growth, causing the refrigerator to have to be defrosted and cleaned more often. Do not place the refrigerator in direct sunlight or close to a heat source. In winter, the temperature in the room where the refrigerator is located should not fall below 100°C: this may impair the operation of the refrigerator. It is not advisable to set up a vaccine warehouse in places that are rarely visited by personnel (for example, once a week) if it is not possible to install an electronic monitoring system. It is better to store supplies in a room where someone is always present, and take a small amount with you each time in a portable refrigerator (so-called camping models that operate on 220 V of a 12-volt car battery and sometimes on gas) or in a good refrigerator box with refrigeration elements. In this case, ensure that there are sufficient frozen refrigeration elements and do not allow them to come into direct contact with the vaccines. It is important to ensure that the refrigerator is not overloaded. To maintain a constant temperature, free air circulation is necessary. Do not place vaccine boxes against the walls; leave approximately 2 cm of free space around them. The refrigerator door and vegetable drawer are not suitable for vaccines because the temperature here is often higher than the rest of the refrigerator. If the refrigerator has a freezer compartment, it should be separated with plastic to prevent ice from forming outside the freezer compartment and freezing the vaccine supply. In the latter case, it is recommended to place lyophilized vaccines as close to the freezer as possible.
Defrosting the refrigerator
While the refrigerator is defrosting, the vaccine stock should also be kept chilled. If there is a second refrigerator at the correct temperature, it is preferable to place the vaccine stock in it. If it is not available, then the vaccines are placed in a refrigerator box with refrigeration elements. You can also leave vaccines in the refrigerator to defrost. In this case, the refrigerator door must be closed during defrosting, and care must be taken to ensure that the thawed water does not wet the vaccines. Set the thermostat control to zero (you should first record the thermostat setting in your diary). With a closed refrigerator without a freezer, any existing ice will melt in about an hour, and the refrigerator can be washed according to the instructions in the usual way (a small amount of water and detergent is almost always sufficient). After washing, the thermostat regulator is returned to its original position and the supply of vaccines is again placed in the refrigerator.
Temperature monitoring: diary
Keeping vaccines in the refrigerator does not guarantee that they are actually being kept at the correct temperature. Careful monitoring of temperature changes in the refrigerator is necessary. If equipment breaks down, it is important to know how long it takes to reach certain temperature indicators. A modern, but expensive way to solve this problem is an electronic temperature monitoring system, which sounds an alarm if the temperature rises or falls beyond preset limits. This system also allows you to detect a power outage in time. If the system breaks down, it generates an alarm, for example a telephone warning is sent to one or more employees. In addition, such a system makes it possible to record the temperature on paper, which allows you to retroactively control how exactly the temperature changed. A simpler and cheaper solution is to read and record the temperature and its extreme values daily (ideally also on Sundays and holidays!). You can use a so-called minimum-maximum thermometer, which is sold at a home improvement store. These thermometers have two bars that indicate the highest and lowest temperatures reached since the thermometer was set to zero. These thermometers should be checked before use and then re-checked periodically, for example once every 6 months. For this purpose, the thermometer can be placed in a jar with water and melting ice; in this case it should show 0°C. If there is a deviation, you should write down in the diary and on the label that is stuck on the thermometer by what amount you need to adjust its readings. The results of each test should also be recorded in a diary. Attention!
Many thermometers contain mercury. If replaced or damaged, mercury thermometers should be treated in the same way as chemical waste.
Using a Minimum-Maximum Thermometer • Place a calibrated minimum-maximum thermometer in the center of the refrigerator, level with the vaccines if possible. • Read the thermometer daily, preferably at the same time. When the mercury column rises, the columns rise to the top and continue to “hang” if the mercury falls again. The bottom of the bars indicates the minimum and the top the maximum temperature. • Record your minimum and maximum temperatures in your diary. • After reading the readings, set the thermometer to zero (most often done by pressing a button between the temperature scales) and place the thermometer back in the refrigerator. Do this quickly (within 1-2 minutes), otherwise the thermometer will heat up too much and the next time it is read, the maximum temperature will be incorrect. • Write down in your diary when cleaning and defrosting were done, when repairs were made and what kind. • Record in your diary when new vaccines are delivered. Be careful as vaccines may be delivered from the same batches as previous ones, and in some cases even older ones. • Conduct a monthly inventory of vaccine stock, recording lot numbers and ensuring that the oldest vaccines are used first. • Remove vaccines immediately after expiration dates.
When is it necessary to take action?
• The refrigerator operates normally as long as the minimum temperature is above 20°C and the maximum temperature is below 80°C. • If a slight deviation is detected (the minimum temperature is above 0°C and the maximum is below 100°C), then the refrigerator should be adjusted and its operation should be constantly monitored. • If the deviation is greater (minimum below 0°C or maximum above 100°C), immediate action must be taken. The cause (for example, a power outage) must be identified and eliminated, and if necessary, a technician can be called. It is better to replace an old refrigerator, because if it is “older” than 10 years, then replacement is often cheaper than repair. Take into account the mistakes of others in your work and take care that they do not repeat themselves. Thus, in one clinic, a cleaner pulled the plug of the refrigerator from the socket in order to turn on the vacuum cleaner, and one employee of the consulting bureau did the same to ensure that the refrigerator did not create interference during a hearing test. Ensure that the plug is securely connected to the socket, for example by securing it with adhesive tape. It is important to have enough outlets for all purposes.
What to do after a “chain break”
If a significant storage failure has occurred, the owner of the vaccines must determine whether the vaccine stock in the refrigerator can still be used. Recommendations for vaccines included in the state vaccination program are given in the table. During transport from manufacturer to consumer, it is not always possible to prevent a momentary break in the cooling circuit, so weigh the advisability of, for example, repacking and postage. Since it is usually not possible to determine whether a vaccine has been exposed to heat during transport, the table provides fairly strict guidelines to ensure good quality. Recommendations for freeze-dried vaccines are based on the assumption that they are stored unprepared for use. Once prepared for use, the shelf life is limited; more detailed information is contained in the insert. Opened multi-dose bottles should always be used within 24 hours and then disposed of normally. When determining the duration of the disturbance and the temperatures reached, it is necessary to assume the worst-case situation. If, for example, on Friday the thermometer showed a normal temperature, and on Monday it turns out that during this time the maximum temperature rose to 21°C, then you need to act on the assumption that the vaccines were at a temperature of 21°C for more than 48 hours, although the likelihood that a well-insulated refrigerator will slowly rise in temperature. The entire stock of vaccines must be replaced, only then can the effectiveness of vaccination be guaranteed.
Conclusion
It is necessary to pay due attention to the organization of vaccine storage. If stored incorrectly, there is a need to destroy vaccines if they are detected in a timely manner (economic damage), and if not detected, there is a high risk of insufficient immunization.
Table. Handling vaccines in violation of storage conditions.
| Duration of violation, h | Vaccine | Extreme storage temperatures, °C | |||||
| <0,0 | 0,0-9,9 | 10,0-14,9 | 15,0-19,9 | >20,0 | |||
| less than 24 | DKSP | A | N | IN | IN | A | |
| Chipboard | A | N | N | IN | A | ||
| EpKK/Hib | WITH | N | N | IN | A | ||
| 24-48 | DKSP | A | N | IN | A | A | |
| Chipboard | A | N | IN | IN | A | ||
| EpKK/Hib | WITH | N | IN | A | A | ||
| more than 48 | DKSP | A | N | A | A | A | |
| Chipboard | A | N | A | A | A | ||
| EpKK/Hib | WITH | N | A | A | A | ||
Note.
DKSP - diphtheria, whooping cough, tetanus, polio;
DSP-diphtheria, tetanus, polio; EpMC/Hib - mumps, measles, rubella/Haemophilus influenzae type B; other vaccines: -liquid vaccines - act as with DSP; -lyophilized vaccines - act as for EPA/Hib. N - no harm; A - no longer use; B - use as soon as possible, and store properly before that; C - the vaccine can be used. The solvent bottle can be used as long as there are no signs of leakage or cracks are visible (microscopic cracks may be caused by water expanding when it freezes). If there are cracks, including microscopic ones, then the contents of the bottle may become infected; in this case it cannot be used. The recommendations given in the article are based on the research results cited in the introduction. By following our tips, you can check whether vaccine storage is well organized or if improvements are needed.
Abstract:
Recommendations are provided for the organization of optimal storage and use of vaccines in daily practice.
Literature:
1. Rumke NS. Good clinical practice in het Rij ksvaccinatieprogramma? Infectieziekten-Bulletin 1993:4:277-9. 2. Onoja AL, Adu FD, Tomori 0. Evaluation of meats vaccination program conducted in two separate health centers. Vaccine 1992:10:49-52. 3. Battersby A, Garnett A. Storage and transport of vaccines. Background and study protocol. Report on a visit to the Netherlands, 17-22 June 1990. Geneve: World Health Organization, 1990. 4. Hunter S. Storage of vaccines in the general practice. BMJ 1989:299:661-2. 5. Keja AE, Van Steenbergen JE. De koude keten: zit het venijn in het staartje? Infectieziekten-Bulletin 1993:4:8-11. This article was previously published in Infectieziekten-Bulletin 1993:4(13):280-4 and Tijdschrift voor Jeugdgezondheidszorg 1994;26(2):21-4. Adapted from Pharmaceutisch Weekblad 1995; 130:704-8 with the permission of the main editors, authors and publishers.
Storage Assessment
According to the rules for vaccination sera (MIBP), all vaccines stored in non-compliance with the temperature regime must be disposed of.
Therefore, the order must be constantly monitored:
- adjust the thermostat well, set the required temperature regime and then in the morning and evening check its compliance with the norm, guided by the data read from sensors and temperature indicators;
- All readings from these devices must be recorded in a special journal;
- Vaccines with a short shelf life should be used first;
- never open the door unless absolutely necessary;
- When there is a power outage, close the door firmly.
Precautionary measures
Regular defrosting of the refrigerator.
This requirement is explained by the fact that a layer of ice exceeding 5 mm negatively affects the stability of the operating temperature. During the defrosting period of the unit, refrigerants are placed in a thermal container with vaccines, avoiding direct contact with them. A newspaper or sheet of cardboard is placed as a separating layer between the vaccine and the refrigerants to prevent the MIBP from freezing.
Checking the tightness of the door.
If ice forms too quickly, you need to take a sheet of paper and press it with the door near the camera. A falling sheet of paper indicates that the door is either not closing tightly or is damaged. This method is used to check the door along its entire length.
Checking the condenser located on the back wall of the refrigerator.
Its condition should be checked periodically to ensure that the heat generated by it is dissipated evenly. If necessary, clean the condenser from dust with a soft brush.
Storage of the most popular vaccines
General indicators: from +2°C to +8°C - completely unsuitable for MIBP types, because they need individual recommendations:
- the yellow fever vaccine does not lose its activity only at temperatures from -12°C to -20°C;
- the anti-poliomyelitis remedy is also preserved at -20°C (although the temperature can be increased to 0°C);
- But in no case should you freeze DPT, tetanus toxoid, Infanrix and other liquid and adsorbed drugs.
Proper storage ensures the widespread use of serums, guaranteeing a healthy life for millions of people.